

Drs. Amit Awasthi and Sweety Samal have been awarded the Intensification of Research in High Priority Area (IRHPA)-SERB grant funded by Department of Science and Technology to support COVID-19 anti-viral research. The project is in partnership with The International Centre for Genetic Engineering and Biotechnology (ICGEB), and Madurai Kamaraj University, Madurai. THSTI will support in conducting animal studies both in mice and hamsters in screening and identification of promising COVID-19 antiviral drugs. The project has been awarded for 3 years till 2023.
Epidemiological and serological surveillance of COVID-19 was conducted in Pune. The participating institutes were IISER Pune, Savitribai Phule Pune University (SPPU), Pune, THSTI, CMC, Vellore, Pune Municipal Corporation. The study is supported by the Persistent Foundation. The survey specifically targeted selected high-incidence prabhags of Pune city. THSTI's in-house Receptor-Binding Domain (RBD)-ELISA assay was used for testing the clinical samples.
IgG antibodies against the receptor-binding domain (RBD) of the viral spike protein was detected using the highly specific (100%) and sensitive (84.7%) THSTI-RBD-ELISA assay. This assay has been extensively characterized and compared with other commercially available tests for SARS-CoV2 IgG. The manuscript has been uploaded on Medrxiv (doi: 10.1101/2020.08.12.20173856).
The preliminary technical report (here) and summary of the initial findings (here) are available on IISER Pune's website.
The COVID19 pandemic has been perceived a disaster. Talk about the scientific community, it has also been a test of resilience. A government issued memorandum issued a month back gave institutes other than those funded directly by ICMR, access to COVID19 samples. This very step has changed the way India does science.
“It makes a huge difference to us. Now we can think about diagnostics. We can think about clinical trials. We can think about anything to do with patients.”, said Dr. Gagandeep Kang, Executive Director, DBT-THSTI, welcoming the government’s memorandum during an interview to The Wire.
Taking up the opportunity, the Department of Biotechnology and National Biopharma Mission at Biotechnology Industry Research Assistance Council (BIRAC), Govt. of India have initiated an effort to understand human COVID-19 infections by announcing the ‘COVID19 Research Consortium’. Following the call, THSTI was identified as the focal center for clinical and translational research on COVID19.
THSTI is a focal center for the DBT research consortium for clinical and translational research on COVID 19 to address the following objectives:
Following its uniquely translational approach, THSTI, in this initiative has built collaborations and rekindled the older ones with various hospitals and diagnostic laboratories to embark on this initiative. Some of them are the Foundation for Innovative New Diagnostics (FIND); Lady Hardinge Medical College (LHMC), New Delhi and associated hospitals; Safdarjung Hospital, New Delhi; Gurgaon Civil hospital; Christian Medical College (CMC), Vellore; Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh; Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry; Ram Manohar Lohiya hospital, New Delhi; Medanta Hospital; BigTec labs; University of Turku, Finland; Civil Hospital, Palwal. Collaborations have already been established with five hospitals and one diagnostic lab. The goal is to build collaborations with various hospitals and diagnostic laboratories in the NCR region for getting well phenotyped clinical biospecimens; take all the requisite ethical permissions from the various hospitals and IBSC approvals and have well trained technical teams for collecting samples.
CURRENT STATUS - Study initiated. Over the next one to two months, more hospitals will be added as the study progresses.
PROGRESS - Collaborations have already been established with 5 hospitals and 1 diagnostic lab. Trained manpower is needed to be protected. The major risk factor is the exposure of the staff which is collecting and handling the clinical samples. All efforts have been made to provide a stable environment with all protective equipment to avoid exposure. The teams have been properly trained on proper practices on the collection and handling of the samples.

Prof. Gagandeep Kang, the current Executive Director of the DBT funded Translational Health Science & Technology Institute has joined the governance of the COVID19 Clinical Research Consortium. She has joined as a member of the Facilitation Committee of the initiative. With this, THSTI becomes the member of this global coalition (https://covid19crc.org/members/). The only other member institution from India is the Christian Medical College, Vellore.
The COVID-19 Clinical Research Coalition aims to accelerate clinical research for COVID-19 in resource-limited settings. The coalition brings together institutions and groups working to fast-track research that will provide evidence on COVID-19 prevention, diagnosis, and case management in such settings. More details can be found on its website www.covid19crc.org.
More details can be found in this commentary on Lancet.
Read about media stories on our work:
https://pib.gov.in/newsite/PrintRelease.aspx?relid=202500
Ray of hope: Government eases restrictions on Indian COVID-19 research by Priyanka Pulla
https://science.thewire.in/the-sciences/covid-19-research-empowered-committee-dst-dbt-niv-testing-kits-hcq-rt-pcr-gisaid/
Indian scientists wage frontline battle against coronavirus
https://www.natureasia.com/en/nindia/article/10.1038/nindia.2020.56

Left to Right – Ankit Gupta, Soon Jyoti Das, Dr. Tarun Kumar Sharma, Anjali Anand, Raj Kumar
The humans have witnessed many a pandemic as diseases transmitted across communities and borders by microbial pathogens. Among these pathogens, throughout the history of pandemics, viruses posed a significant threat to public health. The recent outbreak of SARS-CoV-2 was declared a global emergency by the World Health Organization (WHO).
Considering the fatality of COVID-19, the disease caused by SARS-CoV-2, appropriate disease management strategies are required to curtail the disease transmission effectively. The development of vaccines or drugs against this novel virus, especially with the pandemic at our doors, has been challenging. More so, with the discovery of potent lead molecules or vaccine itself being both time taking and tedious processes. Rapid and accurate screening/detection of the population for the presence of infection may help in containing the disease. This requires a rapid and accurate diagnostic test.
To address the existing diagnostic gap for an antigen-based SARS CoV-2 detection assay, Dr. Tarun Kumar Sharma and his team at THSTI has developed a panel of SARS CoV2 spike binding aptamers. The panel was designed using the highly diverse (with 1015 unique molecules) aptamer library at THSTI. The spike protein was expressed and purified by Dr. Tripti Shrivastava also a scientist at THSTI.
Aptamers rival antibodies as their generation do not require an animal. Being a synthetic molecule, an aptamer does not show batch-to-batch variation. This means it can be easily scaled up. Aptamers developed by Tarun and his team can be used to detect the spike protein of SARS CoV2 in nasopharyngeal and oropharyngeal swabs and saliva. These aptamers are being adapted to an appropriate diagnostic platform for rapid and accurate detection of SARS CoV2 infection.
It is evident from the current situation that there is an urgent and unmet need to develop a rapid antigen detection test for SARS CoV2. The aptamer technology might be one of the answers.
The SARS-CoV2 has a spike protein (S), hemagglutinin-esterease dimer (HE), a membrane glycoprotein (M), an envelope protein (E) a nucleocapsid protein (N) and RNA. Spike protein has many glycosyl groups. It utilizes an N-terminal signal sequence to gain access to the endoplasmic reticulum (ER) and attach with host receptors. The spike protein gets its name from its spike-like appearance on the surface of the virus when visualized under a very high-resolution electron microscope. The development of vaccines and therapeutics has taken center stage in clinical research owing to the public health emergency that COVID-19 brought in. For this, it is necessary to evaluate neutralizing antibodies and screen for antiviral drugs or virus entry inhibitors.
Sweety and her colleagues at THSTI have developed a pseudovirus to act as a model to understand how SARS-CoV2 infects - cell entry, trafficking, or induction of neutralizing response. A pseudovirus is a virus particle that has a backbone of a retrovirus that can integrate the structural glycoprotein of the SARS-CoV2 virus, mainly the spike protein. The genome retains the characteristics of the retrovirus itself. Given the infectivity and high pathogenicity of SARS-CoV2, the team works in a Biosafety Level 3 (BSL3) facility. Pseudoviruses are, however, safe and convenient to work in a BSL2 lab. “We aim to produce a pseudovirus bearing the full-length spike (S) protein of SARS-CoV2 in the Env-defective, luciferase-expressing HIV-1 backbone,” says Sweety. “We then established a pseudovirus-based neutralization assay to screen and evaluate neutralizing antibodies, antiviral drugs, and potential vaccine candidates. Besides, we are asking some basic questions about how different domains of the spike protein of SARS-CoV-2 are modulating viral binding to host cell receptors, entry, and trafficking.”
The team comprises Drs. Sweety Samal, Shubbir Ahmed, Rajesh Kumar, Naveen Yadav, Adarsh Chiranjivi, Manish Bansal, Tripti Shrivastava. The team at THSTI is collaborating with the Indian Institute of Science, Bengaluru, and Pentavalent Biosciences Ltd. for this work.

THSTI's biorepository, a core facility, has risen to the COVID-19 challenge posed to the Indian scientific community and is set to support clinical and translational research as a national bioresource.
The biorepository was founded to cater to the GARBH-Ini program of THSTI and other intramural and extramural maternal and child health research projects. The institute's reconstitution last year threw the challenge of opening up to other programs in the NCR Biotech Science Cluster. It all turned out for the best as the core facility was well equipped to step up to the COVID-19 challenge.
The goal that the biorepository is working towards is the development of a national resource of well-phenotyped clinical samples to enable research on COVID-19. This will support researchers to conduct seroepidemiology, immunology, and diagnostic studies with high-quality and well-characterized clinical samples. Samples will be made available for the validation and discovery phase of vaccine and diagnostics development. The repository will comprise of longitudinally collected biospecimens from enrolled participants at three time points within a year, including convalescent samples post-infection. Currently, samples are being collected from nine different hospitals. Building technical teams to process and store infectious samples as per the global and national guidelines is also a focus and a step forward in capacity building at the biorepository. The data management team at the institute is taking care of data entry and curation along with the biorepository teams.
Guidelines for researchers on biospecimen/data sharing SOPs, sample access request forms are now available at https://thsti.res.in/covid_bioresources.php.
The GARBH-Ini team is working with the other clinical research teams to enable the clinical activities of DBT and BIRAC's COVID research consortium for developing diagnostics, vaccines, and therapeutics against COVID. "It has been more than two weeks that @Garbhinicohort teams have been on the frontline for COVID activities. Their dedication and urge to contribute is admirable. It keeps us going.", tweeted Prof. Shinjini Bhatnagar.
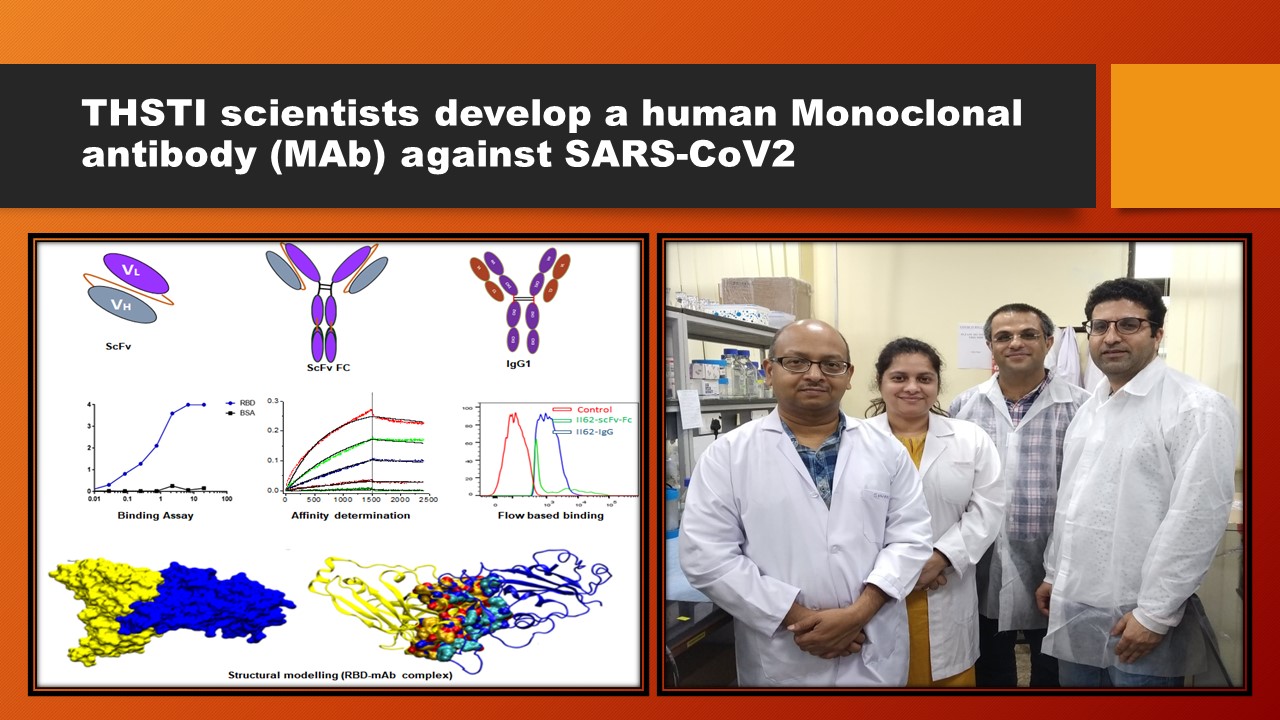
A team of Scientists from DBT-THSTI’s Infection and Immunity Program, led by Dr. Rajesh Kumar, developed the human monoclonal antibody (mAb) against the receptor-binding domain (RBD) of SARS-CoV2. The mAb has been shown to bind to the receptor-binding domain (RBD) protein of SARS-CoV2 with high affinity and specificity. The RBD of the spike protein of SARS-CoV2 is the primary target for neutralizing antibodies to block infection. The team hopes this ‘in-house’ mAb facilitates COVID-19 research activities across the country. The team has developed different formats of this mAb - scFv, scFv-Fc, and IgG1 against COVID-19. This well-established platform allows isolating neutralizing antibodies against COVID-19, which might be helpful to use as an alternate therapeutic.
Furthermore, the availability of an extensive array of SARS-CoV2 specific monoclonal antibodies can help to design vaccines through structural vaccinology. This has been published in the Journal of Biological Chemistry. Read the paper here. The research is funded by DBT.
The other co-investigators in the project are Drs. Shubbir Ahmad, Sweety Samal, Tripti Shrivastava, Hilal Ahmad, Shailendra Asthana, Chandresh Sharma, Shailendra Mani, and Adrash Chiranjivi.
Read R. Prasad's coverage of the aptamer-based assay developed by Dr. Tarun Kumar Sharma and the team.
The COVID-19 pandemic caused by the new coronavirus has resulted in 11,739,167 cases and 540,660 deaths globally, causing panic and significant economic damage across the world. Researchers all around the world are racing to develop a vaccine and antiviral drugs. Currently, most SARS-CoV2 vaccines under development focus on inactivated SARS-CoV2 or its full-length spike (S) protein. However, based on the learning and experience of SARS, there are concerns about the induction of harmful immune responses or inflammatory reactions against non-neutralizing epitopes.
DBT-THSTI’s team, led by Dr. Sweety Samal and Dr. Shubbir Ahmed worked under the supervision of Prof. Gagandeep Kang. The team has identified and synthesized synthetic peptides. These peptides target neutralizing B-cell or T-cell epitopes for developing antibodies against SARS-CoV2. This elicits a desirable immune response that could block viral invasion. The preliminary non-clinical studies in mice are promising. They have further developed a multiepitope-based vaccine candidate by stitching the desired peptides to produce the protein. This approach is unique as it generates potential neutralizing antibodies, while also reducing antibody-dependent enhancement or pulmonary immune pathology, a hallmark of SARS-CoV2 infection.